
Cold Turkey Twice as Effective as NRT or Zyban
All real-world performance studies to date suggest that expensive nicotine replacement therapy (NRT) products like the nicotine patch, gum and lozenge are a total waste of money. In none have NRT quitters performed better than those who quit smoking without them. Now, a May 2006 study survey has found cold turkey success rates twice as high as among those relying upon the nicotine patch, gum, inhaler or bupropion (Zyban and Wellbutrin).
Published in the May 2006 edition of Addictive Behaviors, the survey analyzed 2002 and 2003 patient quitting method data collected by 1,000 Australian family practice physicians. Patients were asked their smoking status, how long since they had last smoked and which of twelve quitting methods they used during their last attempt.
The study established success rates for each of twelve methods by "dividing the total number of successful patients (former smokers) by total number of patients attempting to quit (former plus current) using that method." In analyzing former smokers, it looked at the quitting method used by each former smoker during their last attempt, regardless of the year in which they quit. In assessing current smokers it looked at their last unsuccessful attempt, so long as it occurred within the prior five years. As shown below, the analysis produced rather high success rates.
Success rates among 2,207 former smokers and 928 current smokers were: cold turkey 77.2% (1,942 former, 575 current); nicotine patch 35.9% (145 former, 259 current); nicotine gum 35.9% (52 former, 93 current); nicotine inhaler 35.3% (12 former, 22 current); and bupropion (Zyban or Wellbutrin) 22.8% (36 former, 122 current).
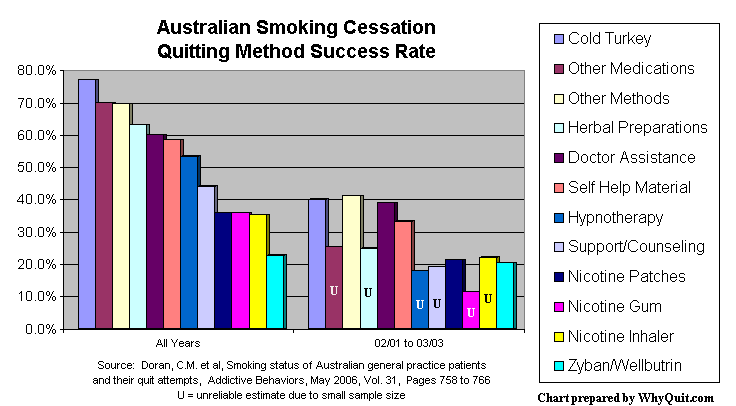
Not only was cold turkey quitting the most effective method -- doubling the rate of pharmacology quitters -- it was by far the most productive method. Successful cold turkey quitters accounted for 1,942 of 2,207 former smokers, a whopping 88% of all success stories.
In theory, all quitting methods should do at least as well as cold turkey. If not, the method has somehow managed to undermine the quitter's own natural ability to break free from nicotine's grip. The Australian study shows that NRT and bupropion clearly undercut a quitter's odds. Other methods surveyed came closer to keeping pace with cold turkey.
Reported rates include: family physician assistance, other than counseling 60.2% (71 former, 47 current); herbal preparations 63.2% (31 former, 27 current); hypnotherapy 53.5% (31 former, 27 current); support/counseling e.g. "SmokeStop," "Quitline" 44.2% (19 former, 24 current); self-help materials e.g. quit smoking manual 58.5% (48 former, 34 current); other methods not listed 69.8% (81 former, 35 current).
What's amazing is that Australian support and counseling programs actually preformed eight percentage points better than the nicotine patch or gum when those products form the cornerstone of Australian quitting efforts. It demonstrates the effectiveness of quality support. When contrasted to cold turkey, it shows that quitters are not succeeding because of NRT but in spite of it.
A Closer Look at Recent Data
The study notes that bupropion (Zyban/Wellbutrin) was not as readily available as other quitting methods in Australia until February 2001 when it joined NRT in its use being subsidized by the government. This fact caused researchers to take a second look at the data, limiting current and former smokers to those making quit attempts between February 2001 and March 2003.
Once again cold turkey quitters clobbered those toying with pharmacology by a two to one margin: cold turkey 40.2% (269 former, 400 current); nicotine patches 21.5% (52 former, 190 current); nicotine gum 11.4% (8 former, 62 current); nicotine inhaler 22.2% ( 4 former, 14 current); and, the reason for the second look, bupropion 20.8% (30 former, 114 current).
Australian Study Will Be Hidden from Smokers
If the true import of this study were ever shared by a major media source it could cost the pharmaceutical industry billions in profits. That's why it will not happen. Like all prior quitting method surveys it must remain hidden from smokers. It will be buried beside a recent survey of UK smoking cessation services, a support based program specifically tailored to assist NRT and bupropion quitters, but in which a few cold turkey quitters managed to squeeze in.
Published in the April 2005 edition of Addiction, the survey of one-year UK program success rates found that 25.5% of cold turkey quitters were still not smoking, compared to only 15.2% of NRT quitters, 14.4% of bupropion users, and 7.4% of those using both NRT and bupropion at the same time.
It will be buried beside a California study in the September 2002 edition of the Journal of the American Medical Association (JAMA) which concludes "NRT appears no longer effective in increasing long-term successful cessation in California smokers." It will join ignored surveys from Minnesota, Quebec, Western Maryland and London, each evidencing zero NRT advantage over those quitting without it.
Evidence Clinical Studies Fatality Flawed
As the volume of real-world survey evidence demonstrating NRT's total ineffectiveness explodes, our understanding of what went wrong in randomized clinical trials of NRT is becoming clearer. Clinical studies assigned participants to one of two groups - those who were to receive the active NRT device being tested (the nicotine patch, gum, inhaler, spray or lozenge) or to a placebo group which was given an identical looking delivery device that was nicotine free.
Generally clinical trials produced results that were exactly the opposite of those found in the Australian survey. Those assigned to the NRT group normally had six month quitting rates that were roughly double those achieved by the placebo group. But how?
Most quitters joined clinical studies in hopes of getting weeks or months of free NRT products. Although NRT studies were supposed to be blind, in that participants would not be able to identify their group assignment, researchers failed to account for the fact that nicotine is a psychoactive chemical producing a dopamine/adrenaline high. Quitters with any prior quitting history knew what it felt like to be deprived of nicotine.
A June 2004 study reviewed blindness in NRT studies and discovered that only 17 of 73 NRT studies took the time to assess blinding, and that 12 of the 17 (71%) failed their own assessment as "subjects accurately judged treatment assignment at a rate significantly above chance."
An April 2005 study published in the Journal of Consulting and Clinical Psychology examined NRT blinding. It randomly assigned smokers who had no intention of quitting during the next six months to use either NRT or placebo. The assigned objective was to try and reduce daily cigarette consumption by half. After six months participants were asked to guess whether or not they had received nicotine or a placebo. Three times as many assigned to the placebo group correctly guessed placebo as guessed nicotine.
Fraud or Slick Nicotine Marketing?
The pharmaceutical industry intentionally keeps smokers in the dark when it comes to both success rates for second time NRT users and the percentage of quitters getting hooked on the cure. Since April 1993 it has known that almost 100% of second time nicotine patch users relapse to smoking within six months. By November 2003 it knew that 36.6% of nicotine gum users were engaged in chronic long term use. Delivering 25% more nicotine, it has every reason to believe that nicotine lozenge dependency rates will go even higher.
There is a grand widening canyon between NRT truth and reality, between clinical efficacy findings and real-world effectiveness. The burning question has quickly become, is it consumer fraud for the pharmaceutical industry and its consultants to continue to tell those addicted to smoking nicotine that replacement nicotine doubles their chances of success, when they know full well that it doesn't?

