
GlaxoSmithKline continues defrauding smokers
Imagine corporate greed so great that it's willing to lie in attempting to destroy all remaining smoker confidence in their own natural quitting instincts, to quit smoking cold turkey.
On October 28, 2010, the day following an announcement that GlaxoSmithKline (GSK) had agreed to pay a $750 million fine in a fraud case, GSK's Consumer Healthcare division issued a press release asserting that, "NRT products offer a step-down therapy that doubles a smoker's chances of quitting versus cold turkey."
It's time to stop being afraid and simply say it. Through and through, GSK's statement is a fraudulent marketing misrepresentation.
Truth is, NRT (nicotine replacement therapy) has failed to prevail over cold turkey quitters in nearly every long-term quitting method survey conducted to date. GSK knows that just last year cold turkey quitters defeated NRT quitters in a survey conducted by four GSK consultants.

More recently, a 2010 United Kingdom (UK) National Health System (NHS) stop smoking program services report found that even as early as 4 weeks into quitting, when NRT quitters still have nicotine circulating in their bloodstreams, and weeks of additional NRT use remaining before needing to adjust to its absence, that those quitting without NRT were actually doing slightly better (see page 59).
The only study of long-term UK NHS quitting program rates was published in 2005 and analyzed success at one-year. It found that at one-year 25.5% quitting cold turkey were still not smoking versus 15.2% of NRT quitters and 14.4% of Zyban (bupropion) quitters (see bottom of Table 6).
The only official U.S. government quitting method survey was conducted by the National Cancer Institute and featured in a front page Wall Street Journal article on February 8, 2007. It followed 8,200 smokers and found that after 9 months cold turkey quitters narrowly prevailed over quitters using the nicotine gum, nicotine lozenge, nicotine patch and Zyban.
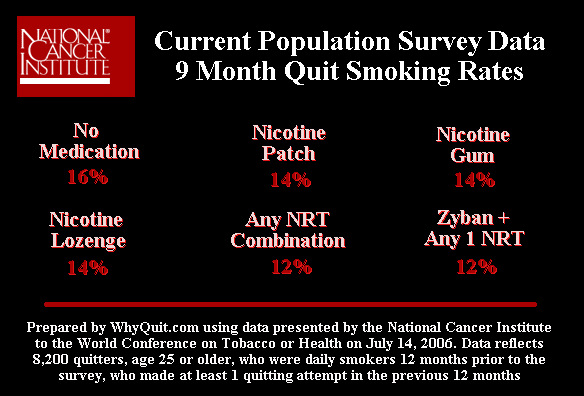
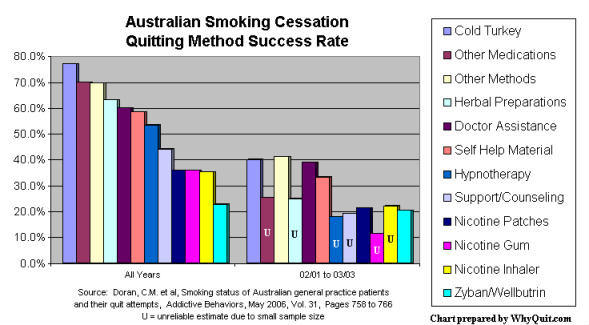
Cold turkey quitters clobbered NRT and Zyban quitters in a 2006 Australian quitting method survey, and an older survey published in the September 2002 edition of the Journal of the American Medical Association concluded that "NRT appears no longer effective in increasing long-term successful cessation in California smokers."
While true that NRT prevails over placebo inside clinical trials, GSK is well aware that cold turkey isn't placebo, and that placebo isn't a real quitting method. Further, GSK knows that the placebo quitter's withdrawal expectations are the exact opposite of the cold turkey quitter's.
Unlike cold turkey, where the quitter fully expects to meet, greet and defeat withdrawal, the placebo group within clinical trials joined the study seeking weeks or months of free replacement nicotine that they hoped would diminish withdrawal's intensity. Instead, they were randomly assigned to inert placebo look-a-likes.

So why do FDA approved quit smoking products prevail over placebo inside clinical trials yet fall flat on their face when going head-to-head against real cold turkey quitters? While placebo controls are the gold standard in most study areas, researchers have known since the very first nicotine gum studies that you cannot blind or hide full-blown withdrawal from experienced quitters who've become experts at knowing exactly how it feels.
Imagine having tried quitting four times previously and then joining an NRT clinical study hoping to receive six months worth of free GlaxoSmithKline nicotine lozenges. Now imagine your frustration upon once again recognizing full-blown withdrawal, and realizing that you'd been given placebo lozenges instead. It's why drug addiction clinical trials measure frustrated and fulfilled expectations, not product efficacy and worth.
Although research
has alerted the pharmaceutical industry to the fact that placebo-controlled 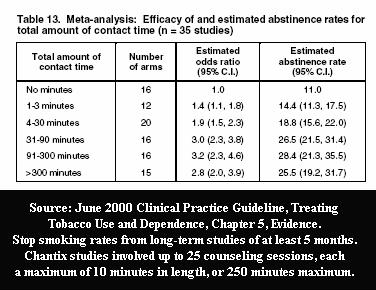 quitting studies are not blind, it hasn't stopped heavy industry reliance upon them. In fact, the most disturbing placebo quitting sham research yet, the NicVax clinical trials, are now nearing completion.
quitting studies are not blind, it hasn't stopped heavy industry reliance upon them. In fact, the most disturbing placebo quitting sham research yet, the NicVax clinical trials, are now nearing completion.
An indispensable ingredient of sham clinical trial quitting research is inclusion of high quality counseling or support, as researchers know that 100 minutes of program contact time can triple success rates.
Most recently, the Pfizer studies that gained Food and Drug Administration (FDA) approval of Chantix involved a record number of counseling/support sessions, twenty-five. Truth is, not even the FDA knows the effectiveness of Chantix as a stand-alone quitting aid, the manner it which it will be used by nearly all users, as there is no such study.
But counseling and support isn't offered in a manner designed to benefit 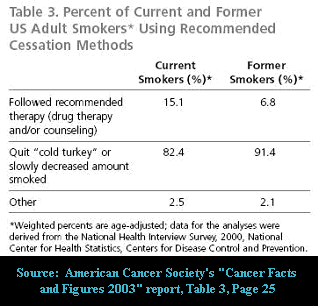 placebo quitters: daily counseling/support through peak withdrawal on the 3rd day, with intense focus upon their first two weeks. Instead, sessions are usually only once a week with content focus primarily upon proper quitting product use, not on how to survive without chemical stimulation of brain dopamine pathways.
placebo quitters: daily counseling/support through peak withdrawal on the 3rd day, with intense focus upon their first two weeks. Instead, sessions are usually only once a week with content focus primarily upon proper quitting product use, not on how to survive without chemical stimulation of brain dopamine pathways.
While GSK clinical trials are engineered for success, out here in the real-world the emperor has no clothes. What GSK has not and will not reveal to smokers is that this year cold turkey will generate far more long-term ex-smokers than all other quitting methods combined. In fact, GSK has spent untold millions attempting to deceive smokers into believing that only super heroes are able to quit cold turkey.
Truth is, although the data in the above American Cancer Society quitting method chart is clearly a bit dated, that out here in the real-world cold turkey remains king.
I, John R. Polito, am solely responsible for the content of this article. Any errors brought to my attention will be immediately corrected.

