Five Facts Chantix Ads Keep Hidden
Critical insights Pfizer doesn't want quitters to know
1. Ray Liotta is an actor who gets paid for pretending
 Pfizer has spent millions on its Ray Liotta Chantix celebrity endorsement campaign. But did Ray Liotta really quit smoking with Chantix?
Pfizer has spent millions on its Ray Liotta Chantix celebrity endorsement campaign. But did Ray Liotta really quit smoking with Chantix?
As reviewed in detail on December 11, 2018, Ray's 2016 social media posts indicate that he quit smoking when his daughter was four, which happened in 2002. The problem is that the FDA didn't approve Chantix for sale until four years later in 2006.
Why care? Because Pfizer is using Ray's tough guy image to plant the seed that if "tough guy" Ray can't quit without spending $1,455 for a three month's supply of a pill exposing him to risks of "aggression, hostility, agitation, depressed mood, or suicidal thoughts or actions," then neither can you.
Should Pfizer have known that Ray claimed to have quit years before Chantix came to market? Absolutely. A simple Google search of Ray Liotta + smoking returns both social media posts. Ask yourself, should Pfizer be trusted?
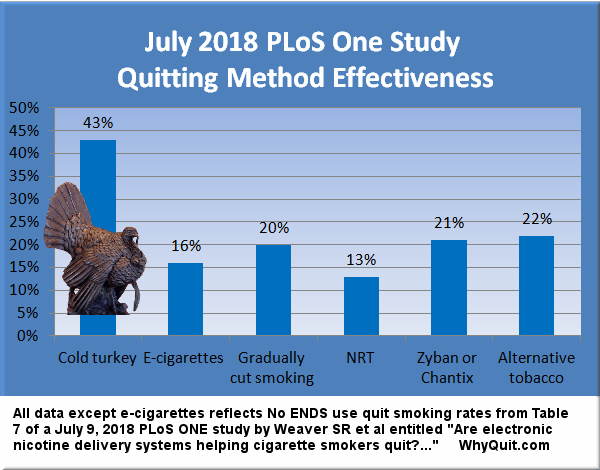
2. Cold turkey doubles your chances versus Chantix
While cold turkey (CT) routinely defeats nicotine replacement therapy (NRT) in real-world quitting method surveys assessing long-term quitting[1], it wasn't until the 2018 PLoS One study that cold turkey and Chantix were analyzed in the same study.
Prior to the PLoS One study we knew that Pfizer's researchers were forced to report that there "were no significant differences" between Chantix and nicotine patch quitters at either 24 weeks (varenicline 38.6% vs. patch 34.1%) or one year (varenicline 34.8% vs. patch 31.4%). The obvious question became, why assume Chantix's long list of serious use risks in exchange for little or no benefit?
That question is vastly more glaring since the PLoS One study. According to Table 7 data, cold turkey was twice as effective as Chantix or Zyban (the study combined their results), and ten times as productive in producing successful tobacco-free ex-smokers.
3. Pfizer used record levels of counseling and support to inflate success rates
No Pfizer advertisement to date alerts smokers to the fact that Chantix drug approval studies set records for the number of counseling and support sessions received by study participants (up to 26). There is a proven dose response relationship between the amount of counseling and support received and successful quitting.
The real mystery is why the U.S. Food and Drug Administration approved Chantix without any idea as to its worth as a stand-alone quitting aid, the manner in which nearly all Chantix is used.
4. Placebo-controlled Chantix studies were not science-based
As a smoker, if participating in a clinical trial and randomly assigned to the study's Chantix group, would you have been able to tell if the dopamine "aaah" sensation that you had come to expect within 10 seconds of a puff was missing, because Chantix was now blocking nicotine from stimulating your dopamine pathways?
If randomly assigned to the placebo group, if you had a lengthy quitting history, would you have been able to recognize the onset of full-blown withdrawal? Hoping for free study medication that diminished withdrawal anxieties, would realizing that you'd instead been given placebo sugar pills have left you frustrated?
Chantix studies were not blind as claimed.[2] Junk science, they reflect fulfilled and frustrated expectations combining with record levels of counseling and support (tailored to benefit Chantix users), not product worth. Use of placebo controls in nicotine cessation studies has been a license to steal. It's why I started a 2009 petition drive asking U.S. health officials to demand honest quitting studies.
5. Full obedience to the "Law of Addiction" provides 100% odds of success
Successful CT quitters discover that it's impossible to relapse and fail so long as no nicotine enters their bloodstream. Success is guaranteed. What Pfizer will never teach smokers is that cold turkey accounts for more long-term success stories each year than all other quitting methods combined.
Cold turkey does not mean quitting without counseling or support. It means ending nicotine use abruptly, without use of replacement nicotine or imitation substitutes.
The body becomes nicotine-free and withdrawal peaks in intensity within 72 hours of ending all nicotine use. The brain works overtime to re-sensitize dopamine pathway receptors and down-regulate receptor counts.
But just one puff of nicotine and up to 50% of nicotinic-type receptors will become occupied by nicotine. Although most who attempt cheating when quitting walk away feeling like they've gotten away with it, it isn't long before they find their brain wanting, plotting to obtain or even begging for more.
Knowledge is Power
Your mind's priorities teacher has been taken hostage. I wish you could spend a few minutes savoring the calm, quiet and comfort inside the long-term ex-smoker's mind. It's why ex-smokers seem so obnoxious. Quitting is often their greatest awakening ever. They simply can't believe how wrong they were.
Think about it. What sense does it make to fear arriving at a day where we go entire days without once wanting to smoke nicotine? Embrace coming home, don't fear it. Healing is good and wonderful not bad. Although almost impossible to believe right now, everything we did as smokers can be done as well or better as us. Recovery is the process of reclaiming life, one activity, person, place and emotion at a time.
 Here's a few key tips for New Year's quitters. Do not skip any meals. If unable to concentrate or experiencing mind fog you've likely skipped a meal. If your health and diet permit, drink extra natural fruit juices but only for the first 3 days (cranberry is excellent). It will aid in helping stabilize blood sugar levels and speed nicotine's elimination from the bloodstream.
Here's a few key tips for New Year's quitters. Do not skip any meals. If unable to concentrate or experiencing mind fog you've likely skipped a meal. If your health and diet permit, drink extra natural fruit juices but only for the first 3 days (cranberry is excellent). It will aid in helping stabilize blood sugar levels and speed nicotine's elimination from the bloodstream.
Caffeine users need to know that ending nicotine use doubles blood caffeine levels. If drinking twice your normal caffeine intake would make you feel anxious or edgy consider cutting your normal daily intake by up to one-half.
Be aware that up to 50% of all smoking relapses are associated with alcohol use. Allow yourself to move beyond peak withdrawal and begin sensing improvement before drinking alcohol. If unable to go three days without drinking you may be facing alcohol dependency issues too. If so, research suggests that arresting both chemical dependencies at the same time likely offers the best odds of success.
Two studies have found that unplanned quitting attempts are twice as likely to succeed.[3] Don't work yourself into a frenzy. Simply jump in the pool. Also, research shows that keeping cigarettes or other nicotine products after quitting increases anxieties and risk of relapse. Although cessation time distortion can make a less than 3 minute crave episode feel like 3 hours, getting rid of all nicotine products builds in relapse delay that just might save your recovery and life.
Visit and explore WhyQuit. Download Joel Spitzer's free e-book "Never Take Another Puff" or my e-book "Freedom from Nicotine - The Journey Home." Watch some of Joel's nearly 500 video quitting lessons, download a detailed quitting tips list, meet Bryan, Noni, Deb and Kim, and visit Turkeyville, WhyQuit's support group.
Knowledge and understanding are key to a lasting recovery. Why quit afraid, alone and in darkness? Why not turn on the lights? Once ready, the next few minutes will be all that matter and each will be do-able. Baby steps! No nicotine just one hour, challenge and day a time. Yes you can!
References:
1. 2013 Gallup Poll; an unpublished 2006 U.S. National Cancer Institute survey of 8,200 quitters, as reported in the Wall Street Journal, Page A1, February 8, 2007; a study of "Smoking status of Australian general practice patients and their attempts to quit"; English smoking treatment services: one-year outcomes published in Addiction (see Table 6); a 2005 study by Alberg AJ entitled Nicotine replacement therapy use among a cohort of smokers; Tobacco In London, Facts and Issues (see Figure 14); a 2002 study by Boyle RG entitled Does insurance coverage for drug therapy affect smoking cessation?, and a 2009 GlaxoSmithKline survey]
2. Mooney M, The blind spot in the nicotine replacement therapy literature: Assessment of the double-blind in clinical trials, Addictive Behaviors, 2004, Volume 29, Pages 673-684, also see, Polito JR Smoking cessation trials, Canadian Medical Association Journal, 2008; also see Polito JR, Widespread Blinding Failures Put Validity of Nicotine Replacement Studies in Serious Question, 2004
3. Ferguson SG, et al, Unplanned quit attempts--results from a U.S. sample of smokers and ex-smokers, Nicotine & Tobacco Research, July 2009, Volume 11(7), Pages 827-832, and West R and Sohal T., "Catastrophic" pathways to smoking cessation: findings from national survey, British Medical Journal, February 2006, Volume 25;332(7539), Pages 458-460.

